An overview of the WHO Essential Medicines List: procedures, usage, and potential improvements
Editorial note
This report is a “shallow” investigation, as described here, and was commissioned by Open Philanthropy and produced by Rethink Priorities from July to August 2022. We updated and revised this report for publication. Open Philanthropy does not necessarily endorse our conclusions.
The primary focus of the report is to provide a review of the WHO’s Essential Medicines List (EML), how it is developed and used, and how it could be improved for a greater global health impact. We reviewed the scientific and gray literature and spoke to three experts on the WHO EML.
We don’t intend this report to be Rethink Priorities’ final word on the WHO Essential Medicines List, and we have tried to flag major sources of uncertainty in the report. We hope this report galvanizes a productive conversation within the effective altruism community about the role of the WHO EML in improving global health. We are open to revising our views as more information is uncovered.
Key takeaways
- The WHO Essential Medicines List (EML), a register of minimum medicine needs for every health-care system, was first published in 1977 to counter a mismatch between health-care needs and the supply of medicines, particularly in low- and middle-income countries (LMICs). In 2007, the EML was expanded, with the creation of the EMLc, a list that includes essential medicines and formulations for children. [more]
- Our best guess is that the total annual expenditures for the EML/EMLc amount to $5 million-$10 million (90% CI), encompassing the creation of the lists as well as supporting activities by the WHO, e.g., the coordination of international efforts to reduce costs of medicines. Our guess is that the financing gap is about one-fourth of its current budget. However, the EML/EMLc are a core function of the WHO, meaning that it does not accept external funding, though it might accept staff secondments. [more]
- The WHO EML/EMLc are jointly revised biennially by a committee of experts who review applications for additions, deletions, or changes to the lists. The committee considers various evidence-based selection criteria, such as therapeutic need, the medicine’s safety, efficacy, public health relevance, and comparative cost-effectiveness. The committee does not appear to use a standardized process for reviewing the evidence, nor specific thresholds for the selection criteria (at least not any open to the public). Medicines can be and are regularly deleted from the EML for a variety of reasons, including new evidence regarding their safety, or changes in regulatory status, market availability, or cost-effectiveness. [more]
- Medicines on the EML/EMLc are listed with their generic name — although not all items on the lists are medicines (e.g., condoms) — along with indications and formulations (i.e., dosage forms and strengths). They are divided into “core” and “complementary” items, with core items encompassing minimum medicine needs for a health-care system, and complementary items encompassing those for which specialized facilities, medical care, and/or training are needed. Some medicines are identified as being representative of a therapeutic class. [more]
- In a convenience sample of 15 medicines recently added to the EML/EMLc, we found that lead times from US Food and Drug Administration (FDA) approval to inclusion in the EML can vary substantially, with an average of ~10 years, possibly due to high evidentiary requirements. Average lead times for medicines on the EMLc appear to be slightly longer than for the EML (e.g., three to six years longer for diabetes drugs). [more]
- More than 150 countries use the WHO EML to compile their own national EMLs, though they adapt the lists to their own needs, with substantial deviations from the WHO EML and often with a lag. The availability of medicines on national EMLs in LMIC health-care facilities is suboptimal, but higher (~62%) than medicines not on the lists (~27%). [more]
- The WHO has a variety of supporting activities and tools for the EML/EMLc, such as support of national medicines policies, coordination of medicine and diagnostic donations, and activities to ensure fair drug prices. [more]
- Many international organizations develop their own EMLs based on the WHO lists, but also provide input for the development of the WHO EML/EMLc. The Global Fund and the Medicines Patent Pool by Unitaid aim to make essential medicines more affordable through price negotiations or voluntary licenses with patent holders. [more]
- A brief literature review and three expert interviews suggest that the current EML/EMLc is likely fairly up to date and complete. Some concerns of missing or underrepresented medicines were raised in the past but have since been addressed by the WHO. Applications for medicines that do not have a corresponding WHO department might be underrepresented. There is a recurrent debate on the role of medicines for rare diseases on the lists, on which the WHO’s stance seems ambiguous. [more]
- We suggest three key areas in which additional funding could be used to improve the EML/EMLc. In particular, funding could be used to improve (1) the quantity and quality of applications to the lists, particularly in relation to the cost-effectiveness information included in the applications, (2) the scrutiny of the WHO’s selection of medicines, and (3) the cross-country coordination and national adaptation of the WHO EML/EMLc. [more]
- We also suggest three lines of further research to resolve key uncertainties:
- Investigating whether and in what form the WHO EML accepts further funding or support.
- Getting a better sense of the EML-related timelines, whether they represent a major bottleneck, and whether this can be solved with more funding.
- Clarifying whether the pool of applications for the EML might adversely skew the medicines listed on the EML, whether the prioritization of medicines can be further improved, and if this is an area that can be influenced with more funding. [more]
The WHO Essential Medicines List was created in 1977 to improve access to essential medicines in low- and middle-income countries
[Confidence: High. Further desk research might add detail, but main elements are captured here.]
The World Health Organization (WHO) Model List of Essential Medicines (Essential Medicines List or EML) is “a register of minimum medicine needs for every health-care system. The idea behind the list is that the use of a limited number of well-known and cost-effective medicines may lead to improved long-term medicine supply, lower costs and better health care provision” (Purgato & Bambui, 2012, p. 1).1
Essential medicines are defined by the WHO as “those that satisfy the priority health care needs of the population” (Executive Board, 109, 2002, p. 3). The WHO EML is not prescriptive, but rather aims to assist countries in developing their own national priority lists of medicines by providing them information on the efficacy, safety, public health relevance, and cost(-effectiveness) (Marks et al., 2017, pp. 637-638).
The first WHO EML was published in 1977 (then called “Model List of Essential Drugs”) and included 186 medicines (Laing et al., 2003, p. 1723). The first Essential Medicines List for Children (EMLc) was published in 2007.2 The EML was introduced as a response to a mismatch between health-care needs and the supply of pharmaceutical products in low- and middle-income countries (LMICs; WHO, 1977, p. 9).3 While by the 1970s, effective medicines existed for most major illnesses, for many LMICs, “modern medicines were unavailable, unaffordable, of poor quality, or ineffective because not properly used” (Quick et al., 2002, p. 913). In 1977, very few countries had national drug policies or essential medicines lists (Quick et al., 2002, p. 913), but a few pioneering countries with essential medicines lists reported favorable medical and economic outcomes (WHO, 1977, p. 9). According to the WHO (2014b), the EMLc was introduced in light of a “growing awareness among regulatory authorities that children were not being specifically catered to in medicines.”
The EML and EMLc are revised every two years.4 We have not been able to find out why they chose this cadence. The most recent EML (22nd list; WHO, 2021d) and EMLc (eighth list; WHO, 2021e) were both published in 2021, containing 497 and 350 medicines, respectively (WHO, 2021b, p. 1). Thus, the number of medicines on the EML has more than doubled since its launch in 1977. The WHO also has an online database with information on the essential medicines, the eEML (WHO, n.d.-c). The eEML is a convenient web interface that shows information on all medicines included on the EML, when and why they were included or deleted from the list, and can also be used to filter, e.g., by year or type of medicine.
In the following, we will refer to both the EML and EMLc as “EML,” as both lists are jointly updated using the same procedure.
The WHO spends $5 million-$10 million per year to produce the EML
[Confidence: Medium. We are fairly certain that more desk research will not provide substantially better information on the funding. This would likely require talking to someone from the WHO EML team.]
During a ~1.5 hour search, we did not find exact funding figures for the EML, neither on the WHO website nor in external sources, such as the scientific literature and the websites of large funders like the Bill & Melinda Gates Foundation (BMGF). However, we found that the WHO categorizes its financial flows by “outcomes,” which are subdivided into “outputs” (WHO, n.d.-h). We are fairly confident that the EML falls under the output category “Provision of authoritative guidance and standards on quality, safety and efficacy of health products, including through prequalification services, essential medicines and diagnostics lists.” We have not found a definitive list of what exactly falls under this output category.
This output category had a total budget of $70 million from 2020-2021, of which $42.8 million was allocated to staff and $27.2 million to activities. Unfortunately, we have not found any information on how this budget was split across the EML and other activities, such as prequalification services or the Essential Diagnostics List. Moreover, we have not seen any breakdown of the funding sources for this category.5 Based on this limited information, our best guess is that the total annual expenditure for the EML is about $5 million-$10 million (80% CI).6,7 We think it is extremely unlikely that the annual budget is above $100 million.
There seems to be room for more funding, as the WHO reported a financing gap of 23% for this output category in 2021 (WHO, n.d.-h).
However, according to Richard Laing (independent consultant, retired Professor of Global Health, and former Medical Officer at WHO), the EML is one of the WHO’s “core functions,” for which the WHO does not accept external funding. He also emphasized that, consequently, large funders, such as BMGF, do not fund the WHO EML.8 All the EML funding comes from the WHO’s regular budget, i.e., from member countries’ contributions. According to Laing, one potential alternative way to support the WHO EML — as direct funding is likely not possible — is through seconding staff. However, as Murray Lumpkin mentioned a current headcount freeze at the WHO, it’s not clear whether seconding staff is a possible option either.9
The WHO does not state how exactly it uses the budget for the EML, but we found that the WHO Essential Medicines List team consists of four regular staff members (WHO, n.d.-f). Moreover, the WHO’s committee of experts who oversee recommendations for updating the EML every two years comprises eight to 12 members (see next section). Apart from the creation of the EML, the WHO carries out a variety of supporting activities, as detailed here. For example, the WHO also coordinates international efforts with the aim of reducing costs of essential medicines (e.g., Schroeder et al., 2018).
The WHO updates the EML every two years, both adding and removing items
Candidate items pass through a three-step process whose selection criteria are somewhat opaque
[Confidence: High.]
Every two years, the WHO publishes an updated version of the EML “to reflect changes in global health concerns, pharmaceutical developments and patterns of drug resistance” (Purgato & Barbui, 2012, p. 343). The process of creating and revising the EML has changed over time. Since 2002, the selection process evolved to become more evidence-based, including an evaluation of the efficacy, safety, cost-effectiveness, and public health relevance of medicines (Laing et al., 2003, p. 1723). The most recent document outlining the WHO’s EML selection procedure is from 2002 (Executive Board, 109, 2002, pp. 6-9).
The selection process follows roughly three steps:
- Application: WHO departments or outside institutions (e.g., academic centers, public or private institutions) can submit applications to suggest inclusions, changes, or deletions to the EML. These applications need to be evidence-based and include an explanation of why a specific medicine or formulation meets (or does not meet) the WHO criteria for inclusion in the EML (Barbui & Purgato, 2014, p. 2).10
In the most recent review, the WHO considered a total of 88 applications11 for the EML and EMLc (WHO, 2021b, p. 1).
- Review: The applications are reviewed by the WHO’s Expert Committee on the Selection and Use of Essential Medicines (henceforth “Expert Committee”), which includes eight to 12 experts from around the world in various fields, such as medicine, pharmacology, and public health. This is an iterative process that involves several Expert Committee meetings and allows for public and WHO commentary on the applications and the proposed changes (Executive Board, 109, 2002, pp. 6-9). The WHO selection procedure stipulates that “specialist assessment(s) are made of the data on comparative efficacy, safety and cost-effectiveness, in close collaboration with relevant departments in WHO” (p. 8). The comparisons of the cost and cost-effectiveness between medicines are “made among alternative treatments within the same therapeutic group, but will generally not be made across therapeutic categories (for example, between treatment of tuberculosis and treatment of malaria)” (p. 9).
Interestingly, in the most recent review, the Expert Committee did not consider any applications related to COVID-19, as the EML “has a longer-term scope and gives much weight to the certainty of the value of selected medicines” (WHO, 2021b, p. 1).
- Publication: Finally, a revised version of the EML gets published. Moreover, the WHO’s decisions to add or delete a medicine or formulation on the EML are narratively reported in an executive summary after the Expert Committee’s meetings (e.g., WHO, 2021b).12 See WHO (2019b) for an overview of additions and deletions of medicines on the WHO EML from 1977 to 2017.
In the most recent review, ~70% of applications for inclusion, change or deletion of medicines were accepted; 20 new medicines were added to the EML, 17 were added to the EMLc, and two medicines were deleted (WHO, 2021b, p. 1). The other changes made relate to, e.g., adding further indications to medicines and changing their dosage form/strength.
The choice of essential medicines depends on several factors, including the disease burden and sound and adequate data on the efficacy, safety and comparative cost-effectiveness of available treatments. Stability in various conditions, the need for special diagnostic or treatment facilities and pharmacokinetic properties are also considered if appropriate. When adequate scientific evidence is not available on current treatment of a priority disease, the Expert Committee may either defer the issue until more evidence becomes available, or choose to make recommendations based on expert opinion and experience (Executive Board, 109, 2002, p. 6).
Our overall impression is that there is no clearly standardized procedure for reviewing the evidence (at least not one that is publicly available),13 and there seems to be some ambiguity in how decisions are made. This impression is shared by some authors (e.g., Barbui & Purgato, 2014, p. 3). This makes the revisions of the EML rather hard to predict, even though the WHO publishes all submitted applications and expert reviews thereof.14
For example, cost has been taken into account as a criterion, but sometimes in rather intransparent and unpredictable ways. Since 2002, the WHO’s EML selection process stipulates that the cost of a medicine does not “constitute a reason to exclude a medicine from the Model List” if that medicine “otherwise met the stated selected criteria" (Executive Board, 109, 2002, p. 4). However, Marks et al. (2017) found that:
In 2011 the Expert Committee rejected the inhalation drug sevoflurane, with the only explanation being “due to cost” — not even unfavorable cost-effectiveness. On the other hand, the Expert Committee added artesunate to the Model List without any consideration of cost analysis because of the medication’s other advantages. (p. 638)
Moreover, the term “priority health care needs” is not a clearly defined concept and leaves some room for interpretation. For example, the WHO does not have a clear selection procedure for medicines for rare diseases. A variety of medicines to treat rare diseases have been added to the EML, such as a cancer drug called “imatinib, which was added to the complementary list despite treating a type of cancer that affects less than 0.001% of the global population annually” and thus arguably has a comparatively low public health relevance (Marks et al., 2017, p. 639).
Existing items can be withdrawn from the EML in light of new evidence
[Confidence: High.]
Medicines can be deleted from the EML for various reasons, e.g., new evidence regarding the harms and toxicity of medicines, changes in their cost or cost-effectiveness, changes in regulatory status (e.g., withdrawal of marketing authorization) or market availability (e.g., discontinuation of products by manufacturers; WHO, 2022b). In each biennial review, the WHO’s Expert Committee decides whether medicines or diagnostics need to be deleted from the lists. The Expert Committee documents the reasons for deletion in executive summaries of its meetings (e.g., WHO, 2021b). The WHO provides an overview of all additions and deletions of medicines on the EML from 1977 to 2017 in table format (WHO, 2019b).
For the most recent EML in 2021, the Expert Committee reviewed “3 proposals for the deletion of 19 medicines or formulations” on the EML and “recommended the deletion of 2 medicines and of specific formulations of a further 13 medicines” (WHO, 2021b, p. 1).
Here are some example statements for recommended deletions in WHO (2021b, p. 4):
- Antituberculosis medicines: “The Committee recommended deletion from the EML and EMLc of various formulations and strengths of amikacin, amoxicillin + clavulanic acid, isoniazid, isoniazid + pyrazinamide + rifampicin, linezolid, paminosalicylic acid and pyrazinamide, noting that they are not optimal formulations and strengths for tuberculosis treatment, in line with recommendations in current WHO treatment guidelines.”
- Antiretrovirals: “The Committee also recommended the deletion of various formulations and strengths of abacavir, atazanavir, efavirenz, lamivudine, lamivudine + nevirapine + zidovudine, lopinavir + ritonavir, raltegravir and ritonavir from the EML and/or EMLc, in line with recommendations in WHO HIV treatment guidelines and the updated Optimal Formulary and Limited-Use list for Antiretroviral Drugs for Children.”
- Other antivirals: “The Committee recommended deletion of oseltamivir oral powder formulation from the complementary list of the EML and EMLc, noting that this formulation is no longer manufactured or marketed.”
Some medicines in the EML have been added and deleted several times. For example, amodiaquine “has been on the Model List since 1977, was removed in 1979, reinstated in 1982 and removed again in 1988 in view of safety concerns in prophylactic use” until it was finally added again in 2003 (WHO, 2003, p. 10).
Each item in the EML is labeled, categorized, and assigned a priority level
[Confidence: High.]
Medicines in the EML are listed with their international nonproprietary name, also known as a generic name, e.g., paracetamol or amoxicillin. They are listed along with formulations (i.e., dosage form and strength), e.g., “Tablet: 100 mg to 500 mg,” and indications (e.g., “Migraine”; see WHO, n.d.-c).
Not all items on the EML are medicines. Serafini et al. (2020) found that in the 21st WHO EML (WHO, 2019c) “a number of diagnostic agents are present (e.g., fluorescein, amidotrizoate, iohexol, meglumine iotroxate, barium sulfate, tuberculin), as well as some medical devices (e.g., condoms, diaphragms, copper-containing devices). … The list also contains blood derivatives and solutions (e.g., water for injection or oral rehydration salts)” (p. 3).
Medicines in the EML are organized by category (and various subcategories) of medication (e.g., “Medicines for pain and palliative care”). See Appendix A for a list of the highest level categories in the 22nd WHO EML (WHO, 2021d). Since 2017, antibiotics in the EML have a slightly different classification system, called the AWaRe Classification, that was introduced to support “antibiotic stewardship at local, national and global levels and to reduce antimicrobial resistance” (WHO, 2021d, p. 7). See Appendix B for further information on this classification.
The EML is divided into “core” and “complementary” items. According to the WHO, “the core list presents a list of minimum medicine needs for a basic health-care system, listing the most efficacious, safe and cost–effective medicines for priority conditions. Priority conditions are selected on the basis of current and estimated future public health relevance, and potential for safe and cost-effective treatment. … The complementary list presents essential medicines for priority diseases, for which specialized diagnostic or monitoring facilities, and/or specialist medical care, and/or specialist training are needed” (WHO, 2021d, p. 3). However, as stated earlier, it is not entirely clear how the WHO defines “priority conditions” and “public health relevance.”
It takes approximately 11 years for a medicine to be added to the EML after US FDA approval
[Confidence: Medium. Below we suggest a possible way to reduce this uncertainty further if desired, which is probably time-consuming.]
First of all, it is important to note that approval by the US Food and Drug Administration (FDA) or European Medicines Agency (EMA) is not a prerequisite for medicines to be added to the EML. Some medicines on the EML are not FDA or EMA approved. For example, delMoral-Sanchez et al. (2020) investigated the availability of authorizations from the FDA and EMA of medicines on the seventh EMLc (WHO, 2019d) and found that ~80% of its studied medicines had commercial authorization from both the EMA and FDA.
We have not found any systematic investigation of the typical lead time between FDA or EMA approval of a medicine and its inclusion on the EML in a ~one hour search. However, we found some evidence that uptake in the EML can be rather slow, though with exceptions.
Serafini et al. (2020) collected information on the FDA approval year of medicines on the 21st EML. They state that there is a “slow uptake in the EML of novel technologies due to the need to gather sufficient evidence. … In recent years, though, drugs that have shown the important magnitude of benefits such as antihepatitis C drugs (e.g., daclatasvir, sofosbuvir, glecaprevir) or cancer immunotherapies for melanoma (e.g., nivolumab, pembrolizumab) have rapidly reached the list” (pp. 10172-10174). While Serafini et al. (2020) provided data on the FDA approval years of 345 medicines on the 21st EML (available in their study’s supporting information), they unfortunately did not provide information on the inclusion years of medicines on the EML. Thus, it is not possible to calculate lead times for medicines based on the authors’ data alone.
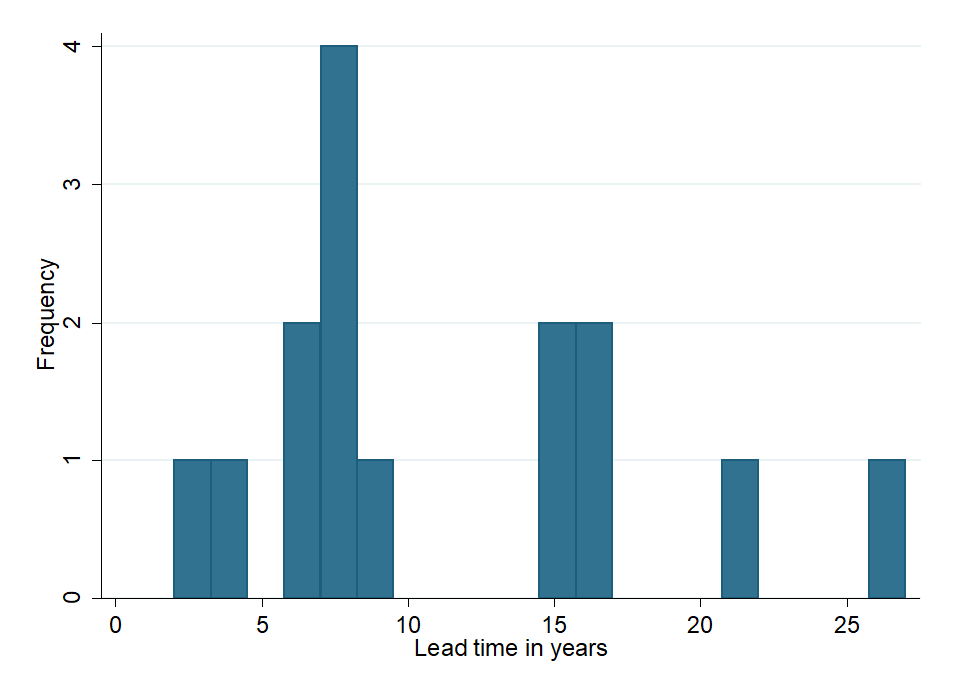
In Appendix C , we spent ~30 minutes collecting a nonrepresentative convenience sample of 15 recently added medicines to the EML or EMLc in 2021 and their respective FDA approval years out of 37 medicines added to the EML and EMLc in 2021 (as mentioned here ). This sample consists of medicines for various indications, such as diabetes, hepatitis C, smoking cessation, Candida infection, and schizophrenia. For this convenience sample of 15 medicines, we found that the average lead time between FDA approval and EML inclusion in 2021 was 11.1 years with a range of two to 27 years . A histogram of these lead times can be seen in Figure 1 below.Our small convenience sample suggests that lead times are slightly longer for medicines on the EMLc than on the EML. Six out of the 15 medicines in our sample were added both on the EML and EMLc, six were only added on the EML, and three were added only on the EMLc. As far as we could tell, the ones that were added on the EML only, have not been listed on the EMLc in any previous year. However, the three medicines that were newly included on the children’s list had been added to the EML three to six years prior. In further research, this analysis could be straightforwardly extended to more medicines to investigate whether the lead times differ, e.g., per disease.
Figure 1 : Histogram of years between FDA approval and EML inclusion of 15 newly added medicines in 2021. Analysis by Rethink Priorities; see Appendix C for data sources.
It is not clear what the reasons for those rather long lead times are. There are several possibilities:
- One possibility is that applications for medicines are submitted long after their FDA or EMA approval and could benefit from a faster application. While we have not examined application dates for medicines systematically, our general impression is that applications are often submitted multiple times before being accepted. For example, long-acting insulin analogues were unsuccessfully considered three times for inclusion in the EML until they were eventually included in the 22nd EML (WHO, 2021b, p. 11).
- Another possible explanation for long lead times are the high data requirements for inclusion of medicines in the EML, as the WHO considers not only efficacy and safety of medicines, but also their public health relevance and comparative cost-effectiveness, which — to our knowledge, though we have not verified this — are not considered for FDA approval. Our best guess is that this is the more likely explanation for long lead times, as Serafini et al. (2020) also pointed out a “slow uptake in the EML of novel technologies due to the need to gather sufficient evidence” (p. 10172).
- Another possible explanation we can think of (low-confidence speculation) is that some important parameters of the medicines change over time, such as their cost(-effectiveness) and public health relevance. For example, according to WHO (2021f), the diabetes burden has been growing over time, which might partly explain the recent addition of diabetes medicines to the EML.
The WHO EML is extensively used by national bodies and international organizations
The EML has been adopted and adapted by national bodies, with uneven success in drug availability
[Confidence: High.]
More than 150 countries consult the WHO EML in devising their national EMLs
The usage of the WHO EML has a number of benefits (some of which we outline in Appendix D), which makes it a useful guide for countries to develop their own national EMLs based on “regional factors such as patterns of prevalent diseases; availability of medicines, treatment facilities and personnel; affordability; and genetic, demographic, and environmental factors” (IMS Institute for Healthcare Informatics, 2015, p. 12). Thus, it is to be expected that countries do not simply copy the WHO EML, but adapt it to their own needs. According to the WHO (2020), “more than 150 countries currently use the WHO list to work out which medicines best meet their national health contexts and priorities, so they can compile their own national essential medicines lists.”
We included a brief case study on how the Nigerian EML is developed and how it relates to the WHO EML in Appendix E , which is based on an interview we conducted with John Ohaju-Obodo, the current chairman of the Nigerian Essential Medicines List.
A team of researchers created a database of the essential medicines lists (Persaud et al., n.d.; see also Persaud et al., 2019) for 137 (out of 195) WHO member countries based on the WHO’s National Essential Medicines Lists Repository from 2017, tracking differences and similarities between the WHO EML and national EMLs around the world. See Figure 2 below for a map of the number of differences between national EMLs and the WHO EML in 2017.
Figure 2: Map of the number of differences between national EMLs and the WHO EML. From “Comparison of essential medicines lists in 137 countries,” by N. Persaud, M. Jiang, R. Shaikh, A. Bali, E. Oronsaye, H. Woods, G. Drozdzal, Y. Rajakulasingam, D. Maraj, S. Wadhawan, N. Umali, R. Wang, M. McCall, J. K. Aronson, A. Plüddemann, L. Moja, N. Magrini, and C. Heneghan, 2019, Bulletin of the World Health Organization, 97(6), p. 398 (https://doi.org/10.2471/BLT.18.222448). CC BY 3.0 IGO.
In their analysis of this database, Persaud et al. (2019) found that:
- “Each national list contained between 44 and 983 medicines (median 310: interquartile range, IQR: 269 to 422)” and that “the number of differences between each country’s essential medicines list and WHO’s model list ranged from 93 to 815 (median: 296; IQR: 265 to 381)” (p. 394). Thus, no single country’s EML overlaps exactly with the WHO EML.
- Recently added medicines to the WHO EML were included in fewer national EMLs than medicines added earlier, which could mean that national EMLs adopted the WHO EML with a lag.
- A country’s GDP per capita was positively associated with the length of a national EML. Moreover, “countries with lower health-care expenditures appear to have omitted more medicines from their lists that are on WHO’s model list (e.g., Angola and Cambodia), and countries with higher health-care expenditures appear to have included more medicines on their lists that are not on WHO’s model list (e.g., Portugal and Slovakia), although exceptions exist (e.g., Sweden)” (pp. 395-396).
- “Amoxicillin was listed by all countries and diazepam, doxycycline, short-acting insulin, salbutamol, and metronidazole were each listed by 99% of countries” (p. 400).
Piggott et al. (2022) built on Persaud et al.’s (2019) work by investigating differences and similarities between the WHO EML and national EMLs for geographical regions and medicine subgroups. They found that:
- The highest similarity between national EMLs and the WHO EML was in Southeast Asia and the lowest similarity was in Europe, measured by the F1 score.1
- “The F1 score was highest for stomatological preparations, (median: 1.00), gynaecological—anti-infectives and antiseptics (median: 1.00), and medicated dressings (median: 1.00), and lowest for 9 anatomical or pharmacological groups (median: 0.00, eg, treatments for bone diseases, digestive enzymes)” (p. 1).
National essential medicine lists have seen broad success but do not always guarantee drug availability
According to Peacocke et al. (2022), national essential medicines lists (NMLs) are “intended to guide public sector procurement and supply, reimbursement schemes, medicine donations, and local production. Moreover, NMLs can aid countries to prioritize medicines and can be used as the foundation for reimbursement schemes and national treatment guidelines (standard treatment guidelines, STG). Medicine use is a key driver of health-care expenditure; thus, implementing a NML can be a strategy for promoting efficient use of health-care resources” (p. 3).
Having a national EML, however, does “not guarantee the availability of essential medicines in health care facilities” (Bazargani et al., 2014, p. 1). For example, two factors that might hinder the accessibility of medicines are medicine stock-outs or high out-of-pocket costs of medicines (Peacocke et al., 2022, p. 4).
Therefore, Bazargani et al. (2014) investigated and compared the availability of medicines that are on national EMLs and those that are not on EMLs at global and regional levels using facility-based surveys in 23 countries. They found that “across all sectors and any product type, the median availability of essential medicines was suboptimal at 61.5% (IQR 20.6%–86.7%) but significantly higher than non-essential medicines at 27.3% (IQR 3.6%–70.0%)” (p. 1). Thus, the authors concluded that the introduction and promotion of EMLs have indeed led to a prioritization of essential medicines, but that their availability is still suboptimal (pp. 6-7).
We have also come across several articles investigating the availability of essential medicines in specific countries or for specific diseases, which came to conclusions roughly in line with Bazargani et al. (2014). For example, Droti et al. (2019) established the availability of essential medicines for women and children in eight countries in sub-Saharan Africa and concluded that the availability of priority medicines in these eight sub-Saharan countries is “unacceptably low” (p. 1).2
The EML is employed by adjacent WHO programs and global health organizations
[Confidence: Low to medium — spent about 2.5 hours researching this question. The landscape of how these organizations interact is quite complex, and we are fairly confident that further research would provide a lot more examples of the various interactions.]
The WHO implements a wide range of activities and tools related to the EML
The WHO conducts a large variety of activities and provides various tools that are related to the WHO EML and aim to promote universal access to health products. In the following, we briefly describe a few non-exhaustive examples of those activities and tools we adapted from the WHO’s “Strengthening Access to Essential Medicines” webpage (WHO, n.d.-l).
- Promoting national medicines policies: The WHO aims to support “Member States to develop, implement and monitor national medicines policies that ensure these products are appropriately prescribed and dispensed, affordably priced and protected against high out-of-pocket expenses for users, and accessible to all countries and regions, particularly within health facilities” (WHO, n.d.-j). To do so, the WHO provides guidance on essential medicines and health technologies through “policy briefs, technical seminars and collaboration with partners and Members” (WHO, n.d.-j). For example, in 2001, the WHO published its guide, How to Develop and Implement a National Drug Policy (WHO, 2001). Moreover, it acts as the secretariat for the Interagency Pharmaceutical Coordination (IPC) group, which supports the development and implementation of medicines policies. We haven’t been able to find information on the activities of the IPC group.
- Donating high-quality medicines and diagnostics for the control of soil-transmitted helminth infections (STH) in children: The WHO manages donations of benzimidazoles and produces and donates diagnostic material to WHO member states to control STH in children. Since 2012, more than 3.3 billion tablets have been provided to countries in need via donations managed by the WHO (WHO, n.d.-b).
- Ensuring fair prices for medicines: In the most recent Expert Committee meeting on essential medicines, the Committee noted that prices of new medicines have continually increased. Some of these medicines were listed on the EML, but have prohibitively high prices. Therefore, the Committee recommended establishing a Working Group to “provide advice to WHO on policies and rules to make highly priced essential medicines more affordable and accessible” (WHO, 2021b, p. 16). For example, the WHO organizes a series of Fair Pricing Forums “to enable stakeholders to discuss options for a fairer pricing system for pharmaceuticals” (WHO, n.d.-d). Moreover, the WHO helps collect data on medicine pricing and therefore developed a tool to collect and analyze data on the price and availability of medicines in facilities (WHO, n.d.-d).
Global health organizations both refer to and provide feedback on the WHO EML
There is a two-way interaction between many international organizations and the WHO EML. Many organizations, such as UNICEF, UNHCR, Médecins Sans Frontières (MSF), as well as other NGOs, develop their own essential medicines lists (IMS Institute for Healthcare Informatics, 2015, p. 1), which are often adapted from the WHO list. For example, MSF’s (2022) Essential Drugs guidelines were revised “in accordance with the most recent WHO list” (p. 11). Moreover, the MSF manual is “not only used by Médecins Sans Frontières, but also in a wide range of other programmes and contexts” (p. 11).
These organizations also provide input to the development of the WHO EML. For example, MSF, as well as various other organizations such as Health Action International and Resolve to Save Lives, gave presentations during the 2019 WHO Expert Committee meeting (WHO, 2019a).
The Global Fund uses the WHO EML to negotiate prices for malaria, TB, and HIV/AIDS medicines (Global Fund, n.d.).3
Unitaid founded the Medicines Patent Pool (MPP) in 2010 to increase the availability and affordability of medicines through voluntary licenses4 with patent holders (Unitaid, n.d.). The MPP is currently expanding its scope to include patented medicines on the WHO’s EML. In 2016, Unitaid also published a study in which it suggests various options that governments could consider to make essential medicines more affordable (Unitaid, 2016).
The WHO EML is in satisfactory shape, but extra funding could enhance existing processes
The EML is likely fairly up to date and complete
[Confidence: Medium. We propose two possible, though likely time-consuming, options to further reduce uncertainty around this question.]
Summary and recommendations for further research:
We tried to go about this question in three different ways: a ~two-hour literature review, three expert interviews, and an analysis of medicines on national EMLs that were not on the WHO EML in 2017. None of these yielded a very clear picture of missing items on the WHO EML. Our overall, though somewhat uncertain, impression is that the WHO EML is fairly up to date and complete.
If more clarity on this question is desired, we propose two avenues for further research:
- Investigate which diseases do not have a corresponding department at the WHO and why. This could give a hint at what medicines might receive fewer applications for inclusion in the WHO EML, as is, e.g., the case for gastrointestinal or fungal diseases.
- Investigate whether it makes more sense to lobby for the inclusion of orphan drugs, i.e., medicines for rare diseases, on the WHO EML, or to compose a separate list.
More detailed discussion of our current impression:
- We searched the literature on missing items on the WHO EML for ~two hours and found several papers pointing out that specific medicines or disease categories were missing or underrepresented on older versions of the WHO EML. For example:
- According to Blaschke et al. (2020), the 21st EML “favors infections and overlooks products for some conditions with a large global disease and economic burden (e.g., diabetes and mental health)” (p. 69).
- According to Foster and Scott (2020), “The medications included in the current lists, along with their formulations and indications for use, do not fully address the needs of children with rheumatic diseases in the context of current approaches to management.”
- According to Hutchings et al.
(2010), medicines for reproductive health were
underrepresented on the EML.
- We asked three experts (Richard Laing, Brendan Shaw, John Ohaju-Obodo)
whether they think specific disease categories, medicines, or formulations on
the WHO EML are currently missing. None of them raised any specific missing
items and there seemed to be an agreement that the current WHO EML is
likely fairly complete.
However, Laing pointed out that there are likely to be more applications submitted for diseases which are represented by departments in the WHO. For example, the WHO does not have a department for gastrointestinal diseases. Laing mentioned a case where a group of students put forward an application for a medicine related to gastrointestinal diseases on the WHO EML, which was accepted.
Moreover, Laing mentioned a recurrent debate on whether orphan drugs, i.e., medicines for rare diseases, are missing from the list. We have not found a recent statement of the WHO EML on whether and to what extent they should be part of the list. Stolk et al. (2006) suggested that “WHO should explicitly include orphan drugs in its policy sphere by composing a complementary Orphan Medicines Model List as an addition to the EML” (p. 1). According to Laing, this proposal was rejected by the WHO. However, Marks et al. (2017) pointed out that some medicines for rare diseases have been added to the EML, such as Imatinib to treat a very rare cancer (p. 639).
- We investigated which medicines are listed on many national EMLs, but not on
the WHO EML. As suggested by Laing, such asymmetries could indicate which
medicines are considered essential by countries but were missed by the WHO.
Thus, we scraped data from a database of national essential medicines lists (Persaud et al., n.d.) for 137 (out of 195)
WHO member countries and created a ranking of the most frequently listed
medicines on national EMLs that are not on the WHO EML.5
The vast majority of the 1,640 medicines were on relatively few national EMLs,23 which could indicate that they are indeed only relevant for specific countries and do not have sufficient global relevance to be included in the WHO EML. About 2% of medicines were on at least half of the national EMLs included in the analysis. This could potentially mean that the WHO EML was not fully complete (at least as of 2017) and was missing some essential medicines. However, it could also be that these medicines were excluded for not fitting the WHO EML inclusion criteria.
We provide an overview of the top 31 medicines24 that were listed on national EMLs but not on the WHO EML in Appendix F. We investigated whether these 31 medicines predominantly represent certain medicine categories of the WHO EML (as listed in Appendix A) and found that they are distributed across 17 (out of 30) medicine categories with no single medicine category dominating the rest.25 We also investigated the reasons for exclusion from the WHO EML for the top five excluded medicines.26 Our understanding is that four out of those five medicines had initially been part of the WHO EML, but were later replaced with medicines that were found to have less severe adverse side effects.27 The remaining one, neomycin, was never listed on the WHO EML, but is currently listed in the WHO’s “Watch” group for monitoring purposes of antibiotics (see Appendix B). Thus, at least based on this very small sample of medicines, we did not find any medicines that were obviously missing from the WHO EML in 2017.
Extra funding could improve three aspects of the WHO EML
In this section, we discuss our top three suggestions on how additional funding could be used to improve the WHO EML. While we only did a very shallow review of these options, we narrowed down the list to, in our view, the most important and tractable options, though we are rather uncertain about this. We have not investigated the neglectedness due to time constraints. We provide a more thorough discussion of various potential improvements of the WHO EML in Appendix G.
Three important considerations:
- As explained above, the EML is part of the WHO’s core functions for which external funding (beyond the regular member contributions) is not accepted. Thus, it is not clear to what extent and in what way the WHO would accept additional support for the EML. Richard Laing suggested that a possible option could be staff secondments. However, it’s possible that this could be impeded by the WHO’s current headcount freeze.
- As described in Appendix G, the WHO EML could be improved at three levels: (1) the development of the WHO EML, (2) its adaptation for national EMLs, and (3) the usage of EMLs for universal health coverage and access to medicines. Our initial impression is that there is a far greater scope for improvement at levels 2 and 3 than at level 1, but these options seem somewhat less tractable and out of scope for this report. Thus, in our suggestions below, we focus mainly on levels 1 and 2, and ignore level 3.
- A related, important point to consider is that improvements at level 1 (i.e., a better WHO EML) might not necessarily translate into better outcomes (e.g., better access to medicines) if problems at levels 2 and 3 persist. We find it plausible that the downstream access to medicines issues might outweigh any improvements made upstream at the WHO or national level. For example, if high out-of-pocket expenditures prohibit the purchase of essential medicines, improving the WHO EML might have a diminished or no effect.
More resources could improve the quality and quantity of applications to the EML
While we have not encountered obvious gaps in the EML in terms of missing or underrepresented medicines, we find it plausible that the pool of applications (and thus of the medicines on the list) might be skewed towards medicines or diseases that have a more active advocacy base. For example, Richard Laing mentioned that applications for diseases which are not represented by a corresponding WHO department might be underrepresented (as explained here). Moreover, the quality of applications to the EML has, at least in some cases, been found to be of poor quality and not fully in line with the EML’s application requirements (as detailed here). Another important issue is that complaints have been raised that the application process is too lengthy and could be discouraging for applicants as “each component requires a separate detailed, complex application” (Magrini et al., 2014).
This all points to a potential for offering support to applicants to improve the quality and quantity of applications to the WHO EML, especially for potentially underrepresented disease categories.
Some concrete examples of how funding could be used:
- Additional funding could be used to identify potentially underrepresented medicines or diseases on the WHO EML and provide financial support for applications related to these medicines.
- If the bottleneck is related to the availability of evidence on the safety, efficacy, public health relevance, or cost-effectiveness of medicines, funding could be provided to improve the evidence base.
We have some uncertainty on the extent to which the application pool is really skewed and improvements to the application base can be made (as mentioned here). Nonetheless, if further research does establish that this is an important bottleneck, improvements could have far-reaching consequences, as more than 150 countries base their national EMLs on the WHO EML and base many decisions on those lists, e.g., procurement and production of medicines, as well as reimbursement schemes (as detailed here). We have not investigated the tractability in detail, but based on some initial thinking we could not come up with major obstacles to this avenue.
More resources could improve the scrutiny process in medicine selection
As discussed in more detail in Appendix G, we find it plausible that increasing the WHO EML team’s staff capacity through staff secondments could lead to improved scrutiny in the selection of medicines, thereby reducing the chance of overlooking medicines with a high added value, as well as decreasing the risk of prioritizing medicines with a low value.
Two concrete examples of how staff secondments could potentially be used:1
- As the WHO Expert Committee has limited expertise in evaluating cost-effective information (as explained here), a health economist or someone else with expertise in cost-effectiveness analysis could help support the Committee in evaluating the applications and assist applicants with advice prior to submission to support a better selection process for medicines in each revision round.
- Additional staff could provide support to applicants for additions, deletions, or changes to the EML to ensure that the minimum standards for applications are met, which is currently not always the case, and for which the WHO currently does not have sufficient human resources.
Improving the scrutiny process in medicine selection and thereby increasing the quality of the WHO EML is important and has large consequences, as the WHO EML is very widely used. We believe that both options are tractable and have a relatively high chance of being accepted by the WHO, as example 1 was suggested by Richard Laing, who worked for the WHO EML for several years, while example 2 was pointed out by WHO EML staff in Magrini et al. (2014). We have not investigated whether any other organizations are currently working to improve the scrutiny of the WHO EML.
More resources could improve cross-country coordination and national adaptation
As discussed in Appendix E for the Nigerian context as a case study, the national adaptation of the WHO EML can be a highly resource-intensive and underfunded process. While the WHO EML is a useful guide for countries, it needs to be adapted to a country’s specific circumstances, e.g., disease burden and national regulations, as well as the availability of medicines. In fact, as we detail here, “most national lists of essential medicines had more than 200 differences compared with WHO’s model list” (Persaud et al., 2019). Moreover, national EMLs tend to adopt the WHO EML with a lag, as confirmed by John Ohaju-Obodo for the Nigerian context and mentioned here, which could potentially be diminished with additional funding.
Furthermore, as we discuss here, the cross-country heterogeneity in national EMLs goes beyond the expected amount of heterogeneity given the countries’ differences, e.g., with respect to disease burden. This suggests that there might be difficulties in the cross-country coordination of national EMLs, which risks a waste of scarce health system resources.
Examples of how additional resources could be used:
- Funding could be provided directly to national EML committees with scarce resources, which might improve the quality and speed up the (sometimes delayed) process of developing national EMLs.
- Another option could be to establish a cross-country coordinating body. This coordinating body could, e.g., track decisions on national EMLs and make these more visible to other countries, or identify regional trends in national EMLs. This could potentially help countries with similar characteristics save resources, and make better and faster decisions on what medicines to include in their lists.2
Both options also seem important, as a faster (and higher quality) development of national EMLs can allow for a quicker adaptation of various national health policies, such as procurement, or reimbursement schemes.
Key uncertainties and suggestions for further research
Funding:
- We are not certain whether and in what way the WHO EML would accept further funding. We suggest checking with someone working at the WHO to see if there is indeed currently a headcount freeze and if the WHO EML would accept staff secondment or any other form of external support.
Timelines:
- We have not been able to find out why the WHO has decided to revise the EML every two years and whether there would be any scope for or benefits to increasing this cadence. Our general impression is that the current review cadence is not a major bottleneck for the inclusion of medicines to the EML, but it might be worthwhile to check this with someone from the WHO.
- It might be worthwhile to investigate the typical lead times from FDA or EMA approval to inclusion of a medicine on the EML for a broader set of medicines and checking whether this differs across, e.g., medicine categories. This could provide insights into whether it might be worthwhile to help speed up some parts of the process, such as applications, or the cadence of Expert Committee reviews.
Pool of applications & selection of medicines:
- It is not very clear what drives applications for inclusion or deletion of medicines on the EML. It is possible that the pool of applications (and thus of medicines included on the EML) is skewed towards disease areas with a more active advocacy base. Further investigation could clarify whether, for example, making funding available to support applicants, or even to create more evidence for underrepresented medicines, would potentially improve the pool of applications.
- We suggest clarifying with someone from the WHO how exactly they define “priority health care needs” and “public health relevance.” A better understanding of this is also an important piece of information to potential applicants, as it seems currently ambiguous whether certain medicines should be included in the EML, e.g., medicines for rare diseases.
- We are not entirely sure why the WHO has decided to not consider any applications for COVID-19 (beyond what we discuss here) and whether that was a reasonable decision. Further research could investigate the details of this decision and whether it could make sense to lobby for the inclusion of medicines to curb epidemics on the EML.
Acknowledgments
Jenny Kudymowa researched and wrote this report. James Hu assisted in revising the report for the public-facing version. James Hu and Laura Duffy obtained data from the WHO’s database of the essential medicines lists and supported the data analysis. Tom Hird supervised the report. Thanks to Aisling Leow, Melanie Basnak, and Marcus Davis for helpful comments on drafts. Further thanks to Bruce Laing, John Ohaju-Obodo, and Brendan Shaw for taking the time to speak with us. Open Philanthropy provided funding for this report, and we use their general frameworks for evaluating cause areas, but it does not necessarily endorse our conclusions.
If you are interested in Rethink Priorities' work, please consider subscribing to our newsletter. You can explore our completed public work here.
References
African Union. (n.d.). African Medicines Agency business plan. Retrieved April 13, 2023, from https://perma.cc/NF9Q-BW98
Barbui, C., & Purgato, M. (2014). Decisions on WHO’s essential medicines need more scrutiny. BMJ, 349, Article g4798. https://doi.org/10.1136/bmj.g4798
Bazargani, Y. T., Ewen, M., de Boer, A., Leufkens, H. G. M., & Mantel-Teeuwisse, A. K. (2014). Essential medicines are more available than other medicines around the globe. PLOS ONE, 9(2), Article e87576. https://doi.org/10.1371/journal.pone.0087576
Blaschke, T. F., Lumpkin, M., & Hartman, D. (2020). The World Health Organization Prequalification Program and clinical pharmacology in 2030. Clinical Pharmacology & Therapeutics, 107(1), 68–71. https://doi.org/10.1002/cpt.1680
Cullinan, K. (2023, March 8). African Union is making ‘good progress’ in setting up its medicines agency. Health Policy Watch. https://perma.cc/NRA4-WP6B
delMoral-Sanchez, J.-M., Gonzalez-Alvarez, I., Gonzalez-Alvarez, M., Navarro-Ruiz, A., & Bermejo, M. (2020). Availability of authorizations from EMA and FDA for age-appropriate medicines contained in the WHO Essential Medicines List for Children 2019. Pharmaceutics, 12(4), 316. https://doi.org/10.3390/pharmaceutics12040316
Droti, B., O’Neill, K. P., Mathai, M., Yao Tsidi Dovlo, D., & Robertson, J. (2019). Poor availability of essential medicines for women and children threatens progress towards Sustainable Development Goal 3 in Africa. BMJ Global Health, 4(Suppl 9), Article e001306. https://doi.org/10.1136/bmjgh-2018-001306
Executive Board, 109. (2002). WHO medicines strategy: Revised procedure for updating WHO’s Model List of Essential Drugs: Report by the Secretariat. World Health Organization. https://perma.cc/6F9A-N9QT
Federal Ministry of Health. (2020). Nigeria Essential Medicines List (7th ed.). https://perma.cc/ZW7P-8Z52
Foster, H. E., & Scott, C. (2020). Update the WHO EML to improve global paediatric rheumatology. Nature Reviews Rheumatology, 16(3), 123. https://doi.org/10.1038/s41584-020-0368-6
Global Fund. (n.d.). Other essential medicines. https://perma.cc/4FEU-DBEE
Hogerzeil, H. V. (2021, June 21). Access strategies by the pharmaceutical industry. Open session, 23rd Expert Committee on Selection and Use of Essential Medicines, Geneva, Switzerland. World Health Organization. https://perma.cc/BS44-C5LF
Holloway, K. A., & Henry, D. (2014). WHO essential medicines policies and use in developing and transitional countries: An analysis of reported policy implementation and medicines use surveys. PLOS Medicine, 11(9), Article e1001724. https://doi.org/10.1371/journal.pmed.1001724
Holloway, K. A., Rosella, L., & Henry, D. (2016). The Impact of WHO Essential Medicines Policies on Inappropriate Use of Antibiotics. PLOS ONE, 11(3), Article e0152020. https://doi.org/10.1371/journal.pone.0152020
Hutchings, J., Neroutsos, K., & Donnelly, K. (2010). Making the list: The role of essential medicines lists in reproductive health. International Perspectives on Sexual and Reproductive Health, 36(04), 205–208. https://doi.org/10.1363/3620510
IMS Institute for Healthcare Informatics. (2015). Understanding the role and use of essential medicines lists. https://perma.cc/5BZ9-JQW6
Jeličić Kadić, A., Žanić, M., Škaričić, N., & Marušić, A. (2014). Using the WHO Essential Medicines List to assess the appropriateness of insurance coverage decisions: A case study of the Croatian national medicine reimbursement list. PLoS ONE, 9(10), e111474. https://doi.org/10.1371/journal.pone.0111474
Laing, R., Waning, B., Gray, A., Ford, N., & ’t Hoen, E. (2003). 25 years of the WHO essential medicines lists: Progress and challenges. The Lancet, 361(9370), 1723–1729. https://doi.org/10.1016/S0140-6736(03)13375-2
Leow, A., Hu, J., and Hird, T. (Forthcoming). An overview of WHO prequalification. Rethink Priorities.
Lo, C. (2019, October 2). WHO’s Essential Medicines List: Discussing innovation and access. Pharmaceutical Technology. https://perma.cc/9R9T-DFFD
Magrini, N., Robertson, J., de Joncheere, K., & Bero, L. (2014). On WHO’s essential medicines process and transparency [Rapid response to “Decisions on WHO’s essential medicines need more scrutiny,” by C. Barbui & M. Purgato]. BMJ. https://perma.cc/W6AD-3BPY
Marks, V. A., Latham, S. R., & Kishore, S. P. (2017). On essentiality and the World Health Organization’s Model List of Essential Medicines. Annals of Global Health, 83(3–4), 637–640. https://doi.org/10.1016/j.aogh.2017.05.005
Médecins Sans Frontières. (2022). Essential drugs - practical guidelines. https://perma.cc/7F9B-3UHT
Moucheraud, C., Wirtz, V. J., & Reich, M. R. (2015). Evaluating the quality and use of economic data in decisions about essential medicines. Bulletin of the World Health Organization, 93(10), 693–699. https://doi.org/10.2471/BLT.14.149914
Peacocke, E. F., Myhre, S. L., Foss, H. S., & Gopinathan, U. (2022). National adaptation and implementation of WHO Model List of Essential Medicines: A qualitative evidence synthesis. PLOS Medicine, 19(3), Article e1003944. https://doi.org/10.1371/journal.pmed.1003944
Persaud, N., Jiang, M., Shaikh, R., Bali, A., Oronsaye, E., Woods, H., Drozdzal, G., Rajakulasingam, Y., Maraj, D., Wadhawan, S., Umali, N., Wang, R., McCall, M., Aronson, J. K., Plüddemann, A., Moja, L., Magrini, N., & Heneghan, C. (2019). Comparison of essential medicines lists in 137 countries. Bulletin of the World Health Organization, 97(6), 394-404C. https://doi.org/10.2471/BLT.18.222448
Persaud, N., Jiang, M., Shaikh, R., Bali, A., Oronsaye, E., Woods, H., Drozdzal, G., Rajakulasingam, Y., Maraj, D., Wadhawan, S., Umali, N., Wang, R., McCall, M., Aronson, J. K., Plüddemann, A., Moja, L., Magrini, N., & Heneghan, C. (n.d.). Global essential medicines. https://perma.cc/7DHW-M4GB
Piggott, T., Nowak, A., Brignardello-Petersen, R., Cooke, G. S., Huttner, B., Schünemann, H. J., Persaud, N., Magrini, N., & Moja, L. (2022). Global status of essential medicine selection: A systematic comparison of national essential medicine lists with recommendations by WHO. BMJ Open, 12(2), Article e053349. https://doi.org/10.1136/bmjopen-2021-053349
Purgato, M., & Barbui, C. (2012). What is the WHO essential medicines list? Epidemiology and Psychiatric Sciences, 21(4), 343–345. https://doi.org/10.1017/S204579601200039X
Quick, J. D., Hogerzeil, H. V., Velasquez, G., & Rago, L. (2002). Twenty-five years of essential medicines. Bulletin of the World Health Organization, 80(11), 913–914. https://perma.cc/J5KZ-9TBL
Schroeder, L. F., Guarner, J., & Amukele, T. K. (2018). Essential diagnostics for the use of World Health Organization essential medicines. Clinical Chemistry, 64(8), 1148–1157. https://doi.org/10.1373/clinchem.2017.275339
Serafini, M., Cargnin, S., Massarotti, A., Pirali, T., & Genazzani, A. A. (2020). Essential medicinal chemistry of essential medicines. Journal of Medicinal Chemistry, 63(18), 10170–10187. https://doi.org/10.1021/acs.jmedchem.0c00415
Spitz, S. (2013). Physicians should be cautious when prescribing diclofenac. Canadian Medical Association Journal, 185(6), 470–470. https://doi.org/10.1503/cmaj.109-4425
Stewart, J. (2019, August 25). Daklinza FDA approval history. https://perma.cc/6HZP-9VGB
Stewart, J. (2020, September 7). Sovaldi FDA approval history. https://perma.cc/ZXA7-VJN3
Stewart, J. (2021, January 28). Mavyret FDA approval history. https://perma.cc/C739-3SS7
Stolk, P., Willemen, M. J. C., & Leufkens, H. G. M. (2006). Rare essentials drugs for rare diseases as essential medicines. Bulletin of the World Health Organization, 84(9), 745–751. https://doi.org/10.2471/BLT.06.031518
U.S. Pharmacist. (2021, April 14). Antihistamines often misused; here’s advice on doing it right. https://perma.cc/GV97-VDKT
Unitaid. (n.d.). The Medicines Patent Pool. https://perma.cc/WW7G-CSKQ
Unitaid. (2016). Ensuring that essential medicines are also affordable medicines: Challenges and options [Discussion paper]. https://perma.cc/VWT7-73WA
United States Food and Drug Administration. (2001, November 20). Drug approval package: Lantus (insulin glargine [rDNA origin]) injection. https://perma.cc/7XWA-F5PW
United States Food and Drug Administration. (2003, September 10). Drug approval package: Prograf (tacrolimus) injection. https://perma.cc/AD4Q-MN4C
United States Food and Drug Administration. (2005a, July 26). Drug approval package: Levemir insulin detemir [rDNA origin] injection. https://perma.cc/6QTQ-XW3H
United States Food and Drug Administration. (2005b, August 24). Drug approval package: Mycamine (micafungin sodium) injection. https://perma.cc/6QTQ-XW3H
United States Food and Drug Administration. (2006, June 16). Drug approval package: Chantix (varenicline) tablets. https://perma.cc/56EF-KXNJ
United States Food and Drug Administration. (2007, March 30). Drug approval package: Invega (paliperidone) extended-release tablets. https://perma.cc/NCL2-QTBC
United States Food and Drug Administration. (2013a, February 27). Drug approval package: Invokana (canagliflozin) tablets. https://perma.cc/KVA3-6SHR
United States Food and Drug Administration. (2013b, November 12). Drug approval package: Afinitor Disperz (everolimus tablets for oral suspension), 2mg, 3mg and 5mg. https://perma.cc/6TG6-HY3C
United States Food and Drug Administration. (2014a, February 11). Drug approval package: Farxiga (dapagliflozin) tablets. https://perma.cc/8KA6-WFMJ
United States Food and Drug Administration. (2014b, September 8). Drug approval package: Jardiance (empagliflozin) tablets. https://perma.cc/Z84N-MNDX
United States Food and Drug Administration. (2015, November 25). Tresiba (degludec) and Ryzodeg. https://perma.cc/ZSB6-GCQX
United States Food and Drug Administration. (2019, November 14). Drug approval package: FETROJA (cefiderocol). https://perma.cc/ZSB6-GCQX
Welch, C. (2014). The composition of WHO’s expert committee on essential medicines needs more scrutiny. BMJ, 349, Article g5211. https://doi.org/10.1136/bmj.g5211
Wirtz, V. J., Hogerzeil, H. V., Gray, A. L., Bigdeli, M., de Joncheere, C. P., Ewen, M. A., Gyansa-Lutterodt, M., Jing, S., Luiza, V. L., Mbindyo, R. M., Möller, H., Moucheraud, C., Pécoul, B., Rägo, L., Rashidian, A., Ross-Degnan, D., Stephens, P. N., Teerawattananon, Y., ’t Hoen, E. F. M., … Reich, M. R. (2017). Essential medicines for universal health coverage. The Lancet, 389(10067), 403–476. https://doi.org/10.1016/S0140-6736(16)31599-9
World Health Organization. (n.d.-a). Daclatasvir. https://perma.cc/ZXA7-VJN3
World Health Organization. (n.d.-b). Donating high‐quality medicines and diagnostics for the control of STH in children. https://perma.cc/YE9P-CSXC
World Health Organization. (n.d.-c). eEML – Electronic Essential Medicines List. https://perma.cc/6AJH-735M
World Health Organization. (n.d.-d). Ensuring fair prices for medicines. https://perma.cc/2GZ2-GP8X
World Health Organization. (n.d.-e). Expert Committee on Selection and Use of Essential Medicines. https://perma.cc/A64R-QR7G
World Health Organization. (n.d.-f). Expert Committee on Selection and Use of Essential Medicines: About us. https://perma.cc/8N8X-H765
World Health Organization. (n.d.-g). Glecaprevir + pibrentasvir. https://perma.cc/88J6-A3KH
World Health Organization. (n.d.-h). Programme budget web portal: About: Key figures. https://perma.cc/4HJ9-EA9C
World Health Organization. (n.d.-i). Programme budget web portal: Financial flow. https://perma.cc/DXK5-WMRZ
World Health Organization. (n.d.-j). Promoting national medicines policies. https://perma.cc/V7LN-9RJ5
World Health Organization. (n.d.-k). Sofosbuvir. https://perma.cc/4DFX-SNVE
World Health Organization. (n.d.-l). Strengthening access to essential medicines. https://perma.cc/YS3H-73TT
World Health Organization. (n.d.-m). WHO antibiotics portal: Groups. https://perma.cc/8LAK-K6FH
World Health Organization. (1977). The selection of essential drugs: Report of a WHO Expert Committee (No. 615; World Health Organization Technical Report Series). https://perma.cc/VCB9-DTMS
World Health Organization. (2001). How to develop and implement a national drug policy (2nd ed.). World Health Organization.
World Health Organization. (2003). The selection and use of essential medicines: Report of the WHO Expert Committee, 2003 (including the 13th model list of essential medicines) (No. 920; WHO Technical Report Series). https://perma.cc/VWJ9-DJ9Z
World Health Organization. (2014a). The selection and use of essential medicines: Report of the WHO Expert Committee, 2013 (including the 18th model list of essential medicines and the 4th WHO model list of essential medicines for children) (No. 985; WHO Technical Report Series). https://perma.cc/TW66-9TDE
World Health Organization. (2014b, March 16). Behind the Essential Medicines List. https://perma.cc/S6C5-GASF
World Health Organization. (2019a). 22nd Expert Committee on Selection and Use of Essential Medicines. https://perma.cc/QLL2-929C
World Health Organization. (2019b). Additions and deletions of medicines on the WHO Model Lists of Essential Medicines: 1977-2017. https://perma.cc/WC2N-UQQV
World Health Organization. (2019c). World Health Organization Model List of Essential Medicines - 21st List, 2019. https://perma.cc/7YFN-YXEC
World Health Organization. (2020, February 27). WHO launches a digital version of its Model list of Essential Medicines (EML). https://perma.cc/SU2X-72C8
World Health Organization. (2021a). 23rd Expert Committee on Selection and Use of Essential Medicines. https://perma.cc/95ZE-AA73
World Health Organization. (2021b). Executive summary: The Selection and Use of Essential Medicines 2021: Report of the 23rd WHO Expert Committee on the Selection and Use of Essential Medicines, virtual meeting, 21 June–2 July 2021. https://perma.cc/A6T5-AWXD
World Health Organization. (2021c). WHO Access, Watch, Reserve (AWaRe) classification of antibiotics for evaluation and monitoring of use, 2021. https://perma.cc/39MD-4PAK
World Health Organization. (2021d). World Health Organization Model List of Essential Medicines - 22nd List, 2021. https://perma.cc/7FHZ-DWMZ
World Health Organization. (2021e). World Health Organization Model List of Essential Medicines for Children – 8th List, 2021. https://perma.cc/Q3QJ-54EN
World Health Organization. (2021f, November 10). Diabetes.
World Health Organization. (2022a). Abortion care guideline. https://perma.cc/LB4Q-522S
World Health Organization. (2022b). Information for applicants preparing a submission for the 2023 meeting of the WHO Expert Committee on Selection and Use of Essential Medicines. https://perma.cc/TMV3-HV8J
Appendix A: List of high-level medicine categories in the 22nd WHO EML
Source: WHO (2021d)
- Preoperative medicines and medical gases
- Medicines for pain and palliative care
- Antiallergics and medicines used in anaphylaxis
- Antidotes and other substances used in poisonings
- Anticonvulsants/antiepileptics
- Anti-infective medicines
- Antimigraine medicines
- Immunomodulators and antineoplastics
- Antiparkinsonism medicines
- Medicines affecting the blood
- Blood products of human origin and plasma substitutes
- Cardiovascular medicines
- Dermatological medicines (topical)
- Diagnostic agents
- Antiseptics and disinfectants
- Diuretics
- Gastrointestinal medicines
- Medicines for endocrine disorders
- Immunologicals
- Muscle relaxants (peripherally-acting) and cholinesterase inhibitors
- Ophthalmological preparations
- Medicines for reproductive health and perinatal care
- Peritoneal dialysis solution
- Medicines for mental and behavioural disorders
- Medicines acting on the respiratory tract
- Solutions correcting water, electrolyte and acid–base disturbances
- Vitamins and minerals
- Ear, nose and throat medicines
- Medicines for diseases of joints
- Dental preparations
Appendix B: Classification of antibiotics on the WHO EML
Antibiotics on the EML are divided into three groups: Access, Watch, and Reserve (adapted from WHO, n.d.-m):30
- The Access group includes first- or second-choice antibiotics that offer the best therapeutic value while minimizing the potential for resistance.
- The Watch group includes first- or second-choice antibiotics that are only indicated for a specific, limited number of infective syndromes and have higher resistance potential.
The Reserve group includes “last resort” antibiotics that should be reserved for treatment of infections implicating multidrug-resistant organisms and should be tailored to highly specific patients and settings.
Appendix C: US FDA approval years of selected medicines added to the 22nd WHO EML and eighth WHO EMLc
The table below shows a convenience sample of 15 medicines added to the 22nd EML (WHO, 2021d) and the eighth EMLc (WHO, 2021e) in 2021 (as listed in WHO, 2021b, p. 19) and their respective FDA approval years.
| Medicine added in 2021 | Added in EML or EMLc? | Indication | FDA approval year (source) |
| Insulin degludec | Both | Type 1 and 2 diabetes in patients at high risk of hypoglycemia | 2015 (FDA, 2015) |
| Insulin detemir | Both | " | 2005 (FDA, 2005a) |
| Insulin glargine | Both | " | 2000 (FDA, 2001) |
| Empagliflozin | EML | Type 2 diabetes mellitus | 2014 (FDA, 2014b) |
| Sofosbuvir | EMLc (added to EML in 2015; WHO, n.d.-k) | Hepatitis C | 2013 (Stewart, 2020) |
| Daclatasvir | EMLc (added to EML in 2015; WHO, n.d.-a) | Hepatitis C | 2015 (Stewart, 2019) |
| Glecaprevir + pibrentasvir | EMLc (added to EML in 2019; WHO, n.d.-g) | Hepatitis C | 2017 (Stewart, 2021) |
| Canagliflozin | EML | Type 2 diabetes mellitus (as therapeutic alternative to empagliflozin) | 2013 (FDA, 2013a) |
| Paliperidone | EML | Schizophrenia | 2006 (FDA, 2007) |
| Everolimus | Both | Subependymal giant cell astrocytoma | 2012 (FDA, 2013b) |
| Micafungin | Both | Invasive Candida infection | 2005 (FDA, 2005b) |
| Cefiderocol | EML | Infection due to multidrug-resistant pathogens | 2019 (FDA, 2019) |
| Dapagliflozin | EML | Type 2 diabetes mellitus (as therapeutic alternative to empagliflozin) | 2014 (FDA, 2014a) |
| Tacrolimus | Both | Organ transplant rejection | 1994 (FDA, 2003) |
| Varenicline | EML | Smoking cessation | 2006 (FDA, 2006) |
Appendix D: Benefits of the WHO EML
We have not searched the literature specifically for the WHO EML’s benefits, but incidentally found a number of benefits mentioned in the literature. We briefly outline a few non-exhaustive examples in the following.
- Increased funding for essential medicines. According to Schroeder et al. (2018), many large funders only provide funds for medicines if they are included in the WHO EML (p. 2).
- Reduced prices of essential medicines. The EML coordinates international efforts with the aim of reducing costs of essential medicines, for example, through pooled procurement, waived import duties, and advanced purchasing (e.g., Schroeder et al., 2018, p. 2). A prime example that we saw mentioned several times in the literature is the price of antiretroviral (ARV) drugs. According to Wirtz et al. (2017), the price of ARV drugs fell from $10,000 per patient per year when it first entered the WHO EML in 2002 to less than $100 per person per year in 2017 as a result of concerted global efforts by various groups, such as activist groups and donor governments (p. 412). We have not investigated to what extent this is a causal relationship.
- Higher availability of essential medicines. A study based on health facility surveys found that medicines from national EMLs in World Bank low-income economies had ~50% availability vs. <10% availability of medicines that were not on national EMLs (Bazargani et al., 2014). We have not investigated to what extent this is a causal relationship.
- Improved quality use of medicines. Holloway and Henry (2015) investigate whether WHO essential medicines policies were associated with a better quality use of medicines (QUM) using survey data on 10 validated QUM indicators on 36 self-reported policy implementation variables from WHO databases for 2002-2008. The authors found that WHO essential medicines policies were associated with a higher QUM.
- Reduced inappropriate use of antibiotics. Holloway et al. (2014) used antibiotic use surveys from 55 countries to investigate whether inappropriate antibiotic use in the public sector was lower in countries that implemented essential medicines policies recommended by the WHO. They concluded that their “findings confirm that countries implementing these policies have less inappropriate use of antibiotics” (p. 9).
- Better health outcomes. We have not encountered any evidence on this benefit, but we find it plausible that the above benefits translate, at least to some extent, into better health outcomes.
Appendix E: Case study — Nigerian Essential Medicines List
We spoke with John Ohaju-Obodo, current chairman of the National Drug Formulary/Essential Medicines List Review Committee in Nigeria, about how the Nigerian EML (NEML) is developed and how it relates to the WHO EML.
He explained that the committee is set up by the federal government of Nigeria and consists of 25 persons from various backgrounds, such as academia and the health sector, including medical and pharmaceutical organizations and institutions. The NEML is revised every three to four years.31 The most recent edition is from 2020, which is the seventh NEML (Federal Ministry of Health, 2020). It is an adapted version of the WHO EML that takes into account local circumstances, such as disease prevalence. He pointed out that the NEML is purely funded by the Nigerian government. Currently, there is insufficient funding for the NEML.
Ohaju-Obodo described the development of the NEML as a very resource-intensive process, as the committee reviews the available evidence for every medicine in consideration, and sometimes adds medicines not on the WHO EML. The NEML selects medicines using the same criteria as the WHO EML, but also applies additional criteria, e.g., medicines need to be approved by the NAFDAC (Nigerian National Agency for Food and Drug Administration and Control).32 Once the revised NEML is published, it is shared with various end users, e.g., government organizations and NGOs. Government organizations are supposed to base their procurement on the NEML, and it is clearly stated that any drug not on the NEML should not be procured. He mentioned that NGOs and other organizations provide some support, but did not go into further detail.
By and large, the NEML is developed independently from national EMLs in other countries, though in some cases, the NEML references documents from other countries. When prompted, Ohaju-Obodo stated that there would be something to gain from more cross-country coordination or region-specific versions of the WHO EML.
The NEML differs from the WHO EML mainly with respect to the number of medicines, but also the types of medicines and structure. For example, the NEML has a stronger focus on infectious diseases and retained some antibiotics that are no longer on the WHO EML. Moreover, some medicines that appear as a first-line drug on the WHO EML are put on the “complementary medicines” list in the NEML due to, e.g., cost constraints.
Ohaju-Obodo mentioned a high variation in the time it takes from a medicine’s approval by Nigeria’s drug regulator to its being added to the NEML, which ranges from 18 months to several years.
Appendix F: Overview of top 31 medicines on national EMLs not on the WHO EML in 2017
Figure 3: Top 31 medicines not on the WHO EML by the number of national EMLs they were listed on in 2017. Bar labels represent indications for medicines. Colors represent high-level medicine categories as defined by the 22nd WHO EML (see Figure 4 for legend). Analysis by Rethink Priorities; data from Persaud et al. (n.d.); data analysis here.
Figure 4: Shares of medicine categories of 31 medicines on national EMLs that were not on the WHO EML in 2017. Categories represent high-level medicine categories as defined by the 22nd WHO EML. Medicines were not weighed by the number of national EMLs they were listed on. Analysis by Rethink Priorities; data from Persaud et al. (n.d.); data analysis here.
Appendix G: More detailed discussion of potential improvements to the EML
In the following, we provide a brief discussion of the potential improvements and critiques of the WHO EML we came across during a literature review and interviews with three experts (Robert Laing, John Ohaju-Obodo, Brendan Shaw). The improvements listed below are not exhaustive, but represent the most salient potential improvements we found in a very limited amount of time.
We grouped the potential improvements from narrow to broad, i.e., (1) the development of the WHO EML, (2) its adaptation for national EMLs, and (3) the usage of EMLs for universal health coverage and access to medicines. Within these categories, we sorted the points roughly in order of importance.
Improvements related to the development of the WHO EML
- Greater scrutiny in the selection of medicines:
Barbui and Purgato (2014) reviewed the medicines for mental disorders on the WHO EML and found a poor quality of applications, with most of the required information lacking. According to the authors, the consideration of low quality applications risks prioritizing medicines with limited value and overlooking those with a high added value. They made several suggestions on how to introduce more scrutiny in decisions on the EML, such as introducing the GRADE approach for applications “to describe the evidence and rate its quality in an ordered and transparent way” (p. 3).
Magrini et al. (2014) from the WHO responded to the above article, stating that some of the issues raised by Barbui and Purgato (2014) indeed reflect internal discussions at the WHO on the EML selection process, but also pointing out that some ideas, like the suggested GRADE approach, are not always appropriate. They also point out that a too long and tedious application process could discourage potential applicants. As a potential way forward, Magrini et al. (2014) suggested that “more interaction between the WHO Secretariat and applicants can ensure that the minimum standards for applications are met; this requires adequate resources for both WHO and applicants.”
Relatedly, Richard Laing pointed out that a major expertise gap in the WHO’s Expert Committee is on cost-effectiveness. Applications are typically reviewed by disease specialists who don’t have sufficient expertise to evaluate cost-effectiveness information. Moreover, he pointed to a study that found the cost-effectiveness information in past applications to be very weak (Moucheraud et al., 2015). He suggested that this deficiency could be resolved through staff secondments to the WHO consisting of health economists with expertise in cost-effectiveness analysis.
- Greater consistency between the WHO EML and the WHO treatment guidelines:
Laing et al. (2003) pointed out a disconnection between the WHO EML and WHO treatment guidelines, which is exemplified by a medicine called amodiaquine (p. 1725). Amodiaquine was added to WHO treatment guidelines but, for somewhat opaque reasons, rejected for inclusion in the WHO EML. Our interpretation is that this inconsistency between the EML and treatment guidelines sends an ambiguous message to countries on whether a medicine is essential or not.
We have seen this critique mentioned several times, but we are not aware to what extent it is currently still an issue, how important it is, and whether it could be improved with more resources.
- Greater transparency in the selection of medicines:
Marks et al. (2017) analyzed the WHO EMLs published since 1977 for their rationale why certain medicines were or weren’t added/deleted from the lists and found that “the application of the criteria of cost and priority status of essential medicines has fluctuated dramatically over the years” (p. 637). As we outline in the main text, the authors found that cost was taken into account as a criterion in rather intransparent and unpredictable ways and the concept of “priority health care needs” is somewhat ambiguous. Following those findings, the authors called for a more transparent and standardized medicine selection process.
Barbui and Purgato (2014) also pointed out that while the reasons for adding or deleting a medicine in the EML are reported, “this text does not clarify the grounds on which decisions are made” (p. 3). They called for a structured template to report the decisions and increase consistency and transparency. Similarly, Welch (2014) called for more scrutiny and transparency in the selection of Expert Committee members.
We have not investigated whether the WHO is willing to improve on transparency, and whether this would likely translate into a higher quality and use of the EML.
Improvements related to the national adaptation of the WHO EML
John Ohaju-Obodo (as detailed in Appendix C) explained that the development of the Nigerian EML is a very resource-intensive process requiring a lot of adjustment of the WHO EML for the local context and an extensive review of the evidence. Therefore, the Nigerian EML is not updated as often as desired (only every three to four years). He explicitly expressed that additional funding would likely help speed up the process of updating the Nigerian EML. We expect that the situation is similar for the national EMLs in other countries, but we have not investigated this in detail.
Piggott et al. (2022), as described in more detail in the main text, found substantial heterogeneity in the way 137 WHO member countries align their own national EMLs with the WHO EML. They argued that, while some variability is to be expected given countries’ differences in, e.g., disease burden, the inconsistent medicines selection choices go beyond the expected amount of heterogeneity. They claimed that those vast differences suggest “limited interest in or difficulties in co-ordinating medicine prioritisation and a high risk of waste of health system resources from low value choices” (p. 4). This point was supported by John Ohaju-Obodo.
According to the authors, an explanation for the misalignment between national EMLs and the WHO EML is that “the rationale for essential medicines selection might not be efficiently disseminated to countries. Relatively little attention has been given by WHO to its role and responsibility related to effective dissemination of its rigorous evaluation of EMs to date. … There is, however, not yet a repository of all decisions made by the Committees over time. We are in the process of developing this repository. This means that member states cannot easily retrieve, appraise and interpret the evidence used for developing the List” (p. 5).
Peacocke et al. (2022) undertook a qualitative evidence synthesis to identify the determinants of the adaptation and implementation of the WHO EML at the country level. This was to better understand the factors that facilitate or hinder the implementation of national medicines lists. They “found that implementation can be facilitated by national medicine selection committees that operate with consultative mandates, clear leadership and oversight, and monitoring and evaluation. Implementation of NMLs also requires harmonization with reimbursement processes and recommended clinical practice. National standard treatment guidelines (STGs) therefore play a crucial role in translating intentions of NML to clinical practice, while legislation, oversight, and monitoring are additional tools for ensuring compliance” (p. 2).
They found the cost to be a major barrier to implementing national medicines lists and that “updating NMLs following biannual global revisions of WHO EML requires significant financial and human resource investment by countries. … These findings suggest that to maximize the value of NMLs, greater investments should be made in different types of institutions that are needed to support various stages along the implementation pathway from global norms to adjusting prescriber behavior” (p. 3).
Improvements related to the usage of the EML for universal health coverage
Many studies document that the implementation of the WHO EML and national EMLs to support access on the ground is limited, and large gaps in the availability and access to essential medicines remain (e.g., Droti et al., 2019; Piggott et al., 2022; Wirtz et al., 2017). Reviewing all the various improvements needed regarding the role of the EML for universal health coverage is beyond the scope of this report. Nonetheless, we would like to point towards a few helpful sources we came across in the literature.
- The Lancet’s Commission on Essential Medicines Policies discussed what progress has been achieved since the introduction of the WHO EML, what challenges remain, and what are potential ways forward to use essential medicines to promote universal health coverage (Wirtz et al., 2017). They also identified five crucial focus areas for which they provided actionable recommendations: “paying for a basket of essential medicines, making essential medicines affordable, assuring the quality and safety of medicines, promoting quality use of medicines, and developing missing essential medicines.” (p. 403).
- We came across several authors discussing the role of the pharmaceutical industry related to the EML. For example, Hogerzeil (2021) suggested at the most recent WHO Expert Committee meeting that the industry needs to contribute to improving medicines access in LMICs, e.g., through intra-country differential pricing. He pointed out that while good guidance for government access policies exists, WHO guidance for the pharmaceutical industry is currently lacking and needed. In particular, he stipulates that guidance should be offered regarding the question, “What should national governments demand from the pharmaceutical industry in support of access planning?” (p. 7).
Other authors raised concerns that pricing conversations spurred by the EML are “overly antagonistic towards drugmakers” (Lo, 2019). In our interview, Brendan Shaw also mentioned that the addition of medicines to the EML is often immediately followed by pressure to reduce medicine prices. He explained that this would create “perverse incentives” for manufacturers, as they are essentially “punished for creating an essential medicine.”
- IMS Institute for Healthcare Informatics (2015) provides various considerations for potential future improvements of the WHO EML, e.g., as related to intellectual property rights, trade laws, procurement, regulatory systems, pricing, reimbursement, and the EML’s role for universal health coverage. We have not looked into any of those issues or recommendations in detail.
Notes
-
See WHO (n.d.-e) for a full definition of “essential medicines” by the WHO Expert Committee on Selection and Use of Essential Medicines. ↩
-
We have not been able to find the number of medicines on the first EMLc. ↩
-
See WHO (1977) for a more comprehensive explanation of the rationale behind the creation of the EML. ↩
-
According to the WHO EML selection procedure, “the Expert Committee…could meet more often if needed” (Executive Board, 109, 2002, p. 6). ↩
-
See WHO (n.d.-i) for a breakdown of the WHO’s general sources of financing. ↩
-
We divided the $70 million figure in half to obtain an annual budget, and (somewhat arbitrarily) assumed that about one-fourth of this amount is allocated towards the EML. ↩
-
Rethink Priorities simultaneously produced two reports: on WHO prequalification (Leow et al., forthcoming) and the WHO Essential Medicines List (this report). To the best of our knowledge, both WHO projects contribute to the same output category, which would suggest a total biennial budget of $70 million, and an annual budget of $35 million. Our current estimate for the two together is $40 million-$50 million per year. We are aware that this is inconsistent, but have deprioritized further refinement for this shallow. ↩
-
An earlier version of this report cited an article by an Oxfam employee claiming that the WHO EML was paid for by BMGF (Kamal-Yanni, 2012, p. 309). Having talked to Richard Laing, we are fairly convinced this was not actually the case. Moreover, Murray Lumpkin from BMGF mentioned that the foundation has not funded the WHO EML since at least 2014, when he started working for BMGF. ↩
-
We have not been able to verify this information with the WHO. ↩
-
See WHO (2022b) for information on applications for the next version of the EML. ↩
-
This includes “40 proposals for the addition of 38 new medicines or medicine classes, 16 proposals for new indications for 32 currently listed medicines, 13 proposals for the addition of new formulations of 19 currently listed medicines, and 3 proposals for the removal of 19 medicines or formulations on the Model Lists” (WHO, 2021b, p. 1). ↩
-
For example, “The Expert Committee recommended the inclusion of fixed-dose combinations of daclatasvir + sofosbuvir, glecaprevir + pibrentasvir and sofosbuvir + velpatasvir, as well as single agent daclatasvir and single agent sofosbuvir to the core list of the EMLc for the treatment of children with chronic hepatitis C virus infection, based on evidence of pan-genotypic effectiveness and acceptable safety” (WHO, 2021b, p. 5). ↩
-
This stands in contrast to the standardized and transparent approach the WHO uses for developing evidence-based clinical guidelines in which they follow the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach as, e.g., used for the Abortion Care Guideline (WHO, 2022a). This approach helps to evaluate and describe the evidence in a structured and transparent way. It is, to our knowledge, not used for the EML. ↩
-
See WHO (2021a) for all applications for the 22nd EML and the eighth EMLc, including expert reviews and public commentary. ↩
-
The F1 score is a harmonic mean “of the sensitivity and precision … as a single measure of performance. Here we use it as a single measure of performance of the national list for positive list entries, with its best value at 1, and worst value at 0. In this context the mathematical property of the harmonic mean tends to give more weight to countries with shortest lists (which often will have better sensitivity and precision), as opposed to the arithmetic mean, which is more impacted by countries with large listings of medicine” (Piggott et al., 2022, p. 2). ↩
-
In particular, they found that “mean availability of 12 essential medicines for women ranged from 22% to 40% and 12 priority medicines for children ranged from 28% to 57%. Generally, availability was higher in hospitals than in primary care facilities; patterns were less consistent for urban versus rural locations, and public versus private facilities” (Droti et al., 2019, p. 1). ↩
-
“The Global Fund works with a diverse group of antiretroviral (ARV) suppliers, many of whom also manufacture specialized essential medicines commonly used by HIV programs. In 2018, we leveraged our position as one of the largest buyers of ARVs in the global health market to establish framework agreements and negotiate references prices for several key, yet often low-volume, essential medicines recommended by the World Health Organization” (Global Fund, n.d.). ↩
-
“Licences negotiated by the MPP permit other pharmaceutical manufacturers to produce generic versions of patented medicines for developing countries that increase competition and help bring prices down. Licences also provide the freedom to develop new treatments, such as paediatric formulations and fixed-dose combinations” (Unitaid, n.d.). ↩
-
See here for our GitHub repository and here for our ranking of medicines that were not on the WHO EML by the number of national EMLs they were listed on in 2017. ↩
-
It is based on the 20th WHO EML, which was published in April 2017. The WHO EML has been updated twice since then. ↩
-
We only checked whether the top 31 medicines on national EMLs in 2017 have been added to more recent versions of the WHO EML. ↩
-
A possible reason for this “delay” in the inclusion of medicines on the WHO EML could be that the WHO EML has more stringent requirements on the evidence base for medicines (related to safety, efficacy, public health relevance, and cost-effectiveness) than some national EMLs. We have not investigated whether that is the case. ↩
-
67% of these medicines are on 10 or fewer national EMLs, and 89% are on 30 or fewer national EMLs. ↩
-
This is an arbitrary threshold. Note that we chose 31 instead of 30 as two medicines were tied for 30th by number of national EMLs listed on. ↩
-
The four most common categories are “Cardiovascular medicines,” “Medicines for endocrine disorders,” “Antiallergics and medicines used in anaphylaxis,” and “Anti-infective medicines” (see Appendix F). ↩
-
We omitted long-acting insulin, as it has been added to a more recent version of the WHO EML. ↩
-
Glibenclamide had been on the WHO EML in the past but was removed in 2013. Our understanding is that it was replaced by a similar drug with less adverse side effects (WHO, 2014a, p. 66). Diclofenac used to be on the WHO EML, but was later removed, likely due to adverse side effects. According to Spitz (2013), there have been petitions to remove it from the WHO EML as it increases cardiovascular disease risk. Aminophylline was “deleted from the Model List because of the availability of safer and more effective alternatives on the Model List” (Jeličić Kadić et al., 2014). Chlorpheniramine was removed from WHO EML in 2013 and replaced with a drug that was found to be safer (U.S. Pharmacist, 2021). ↩
-
A higher staff capacity could also be used to speed up the revision process of the WHO EML. However, we did not include this option here, as the frequency at which the EML is revised did not strike us as a major bottleneck during our research. ↩
-
The African Medicines Agency (AMA), a “specialized health agency of the AU [African Union] to ensure regulatory harmonization of medicines across Africa” is currently in the process of being operationalized (Cullinan, 2023). We have skimmed the business plan (African Union, n.d.) of the AMA, but we are not aware of how the AMA intends to interact with the WHO EML or national EMLs. ↩
-
See WHO (2021c) for the WHO 2021 AWaRe classification database. ↩
-
He mentioned that the goal is to revise it at least every three years, but it’s not always possible due to logistical and resource constraints. ↩
-
According to Ohaju-Obodo, there are exceptions in cases where medicines are needed urgently and medicines can be added to the NEML prior to NAFDAC approval. ↩